Next steps in driving forward the field of nanomedicine: A catch-up with the Leslie lab
The original version of this article was written by Emily Cooke at the Michael Smith Laboratories (MSL) here on May 21st, 2025.
The lab of Dr. Sabrina Leslie (MSL, Physics and Astronomy; SBME affiliate member) brings together researchers and approaches from physics and biology to drive forward the field of nanomedicine.
Their work involves the use of their high-resolution Convex Lens-induced Confinement (CLiC) imaging technology to look closely at lipid nanoparticles (LNPs), which can act as delivery vehicles in therapeutics like the COVID-19 mRNA vaccines.
By watching the interactions of these LNPs with cells at the single-particle level, their work can provide insight into the efficiency of nanomedicine release, how to pack more ‘medicine’ into a single particle, and how the process of formulating these LNPs can affect their structure and function. All of this information can help with both the development of new therapies that use LNPs as a delivery vehicle, as well as making existing ones even more efficient and effective.
We decided to catch up with some of the trainees in the lab to talk about some of their recent findings, what they enjoy about their work, and also some of the challenges they face in their research.
Albert Kamanzi (AK) is a postdoctoral fellow in the lab with a background in physics, and his work focuses on single-particle imaging across multiple channels to see how particle structure and fusion is affected based on the conditions used when formulating them. He set up the wide-field CLiC imaging microscope in the Leslie lab with support from a Mitacs fellowship.
Erik Olsen (EO) is also a postdoctoral fellow in the lab, who’s currently measuring light scattering properties to count biomolecules inside LNPs, as he looks to open doors for future work to follow particles over extended periods of time. With a background in engineering, his work has focused on data interpretation, and has been supported by a VIF fellowship from Sweden.
Eric Boateng (EB) is a PhD candidate in the Genome Science and Technology Graduate Program who works on single-particle and single-cell imaging for monitoring LNP-cell interactions. With a background in biophotonics, he set up the multi-colour fluorescence and iSCAT confocal scanning microscope in the Leslie lab. He also holds a Mitacs fellowship.
Yao Zhang (YZ) is PhD candidate who works on formulating the LNPs that the lab uses across their research, as well as advancing the development of new formulations for disease treatment. He holds a CIHR fellowship and earned his B.Sc. in chemistry before joining the Leslie and Cullis labs through the School of Biomedical Engineering Graduate Program.
What do you find most interesting about what you’re working on?
AK – I find it most interesting trying to find ways to solve challenging problems through single particle imaging. I enjoy developing assays and methods to solve problems that are unique to this field.
EO – I find it interesting to look at multiple parameters, or properties, of particles at the same time, to see how those different parameters can be related and have effects on one another, and what opportunities these present for improving therapeutics.
EB – I enjoy developing and making accessible new technologies for single-cell imaging and single-particle characterization. These advanced tools open doors to correlating our imaging data with omics work, helping to pave the way for the future of nanomedicine.
YZ – There’s great clinical potential for what we’re working on, which has been very interesting to explore. I also find that there are so many new parameters that haven’t been investigated before that can have profound impacts therapeutically, and having the chance to look closer at this using our CLiC technology is a unique opportunity we get in our lab.
Can you tell me more about your recent publication in ACS Nano?
AK – The particles that we’re studying are initially formulated by rapidly mixing them at low pH. We then have to bring the pH up to physiological conditions to match the conditions of the human body and to stabilize the LNPs, which requires what we call a buffer exchange. This exchange can lead to structural changes in the particles, so in this paper we were using CLiC imaging in real time to investigate how exactly this buffer exchange process affects the final particle formulations.
YZ – This buffer exchange aspect of the manufacturing process is often overlooked. This study helps fill this gap, as it gave us insight regarding how each condition we changed could affect the formulation, and the impacts of those conditions on particle size, morphology, and other physiochemical characteristics.
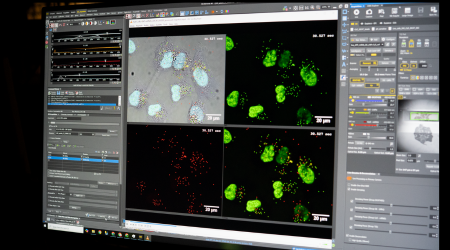
A computer program displays four images taken from the lab microscopes. The images show larger, irregular structures that represent cells, while scattered around and inside the cells are smaller dots that show the LNPs interacting with the individual cells.
What challenges have you faced, or are you facing in the lab with your work?
EB – A challenging part, I will say, is dealing with experimental failure, which happens to everyone. It can also be particularly challenging because we’re generating unique images and videos that we have not seen before. To truly understand and interpret this data relies on collaborations with experts from other disciplines. It’s great, though, that the MSL community brings valuable opportunities and talented collaborators to connect with and make this kind of interdisciplinary work possible.
EO – To build on that, I personally bring a physicist mindset to the lab, and don’t necessarily have a background in analytics for understanding what all of our measurements mean. This is why I really enjoy the cross-disciplinary aspect of our research, getting to work with different departments and labs to bring in those other perspectives to see what our findings mean in practice.
How do you overcome these challenges?
EO – I think we really like to lean into the collaboration, and understanding that we’re not all coming from the same background. When we encounter issues, we have discussions and come together to solve those specific issues, leveraging everyone’s individual expertise.
Where is your research heading next?
EO – We’re moving towards measuring dynamics. The field has been focused for a while on more static, time-specific snapshots of particles, but the next step would be to see how these particles interact in the dynamic environment of the human body. We’ll be able to push the boundaries of current technologies to see how long we can measure particles for, and what we can do with that information for addressing new questions in the field.
YZ – For small molecule drug delivery, the focus will be getting LNPs to release drugs in a timely manner. Something we see especially with chemotherapy drugs, is that cancer patients often have severe side effects from the drugs circulating throughout their entire bodies resulting in healthy tissue damage. We’re working to develop ways to trigger drug release upon command at target sites, breaking open the particles to release their cargo only where it’s needed. This could look like gold nanoparticles that can burst open at a tumour site in the presence of an external stimuli (e.g. laser), delivering cancer drugs directly to their target area. For gene delivery, current LNP formulations result in low mRNA loading on a per particle basis. However, we’re now working towards generating LNPs that are highly loaded with mRNA drugs through the manipulation of manufacturing conditions.
Zhang looks closely as he holds up a small tube, adding liquid to the tube using a pipette.
What are the possible applications of your lab’s research?
YZ – We have already seen approved vaccines for COVID-19 using LNPs, and I do think we’ll likely see an LNP-based cancer vaccine in the next couple of years, if all goes well. With RNA therapeutics, though, you can express virtually any therapeutic protein, which unlocks a lot of opportunities. I think there are many genetic disorders that could be treated this way, such as Huntington’s Disease. There could also be applications in combatting other viruses and pathogens in the future. There is some really great, practical potential for this technology, which is exciting.
Learn More:
- About the Leslie Lab: https://leslielab.msl.ubc.ca/
- About the Michael Smith Labs (MSL): https://www.msl.ubc.ca/
- About biophysics at the UBC Department of Physics & Astronomy: https://phas.ubc.ca/biophysics