|
| Home | Research | Publications | Prospective Students | Teaching | Contact | Physics | Resources | Fun |
| Research Home
Current Projects Other Projects |
Role of Cosolutes in Protein Stability
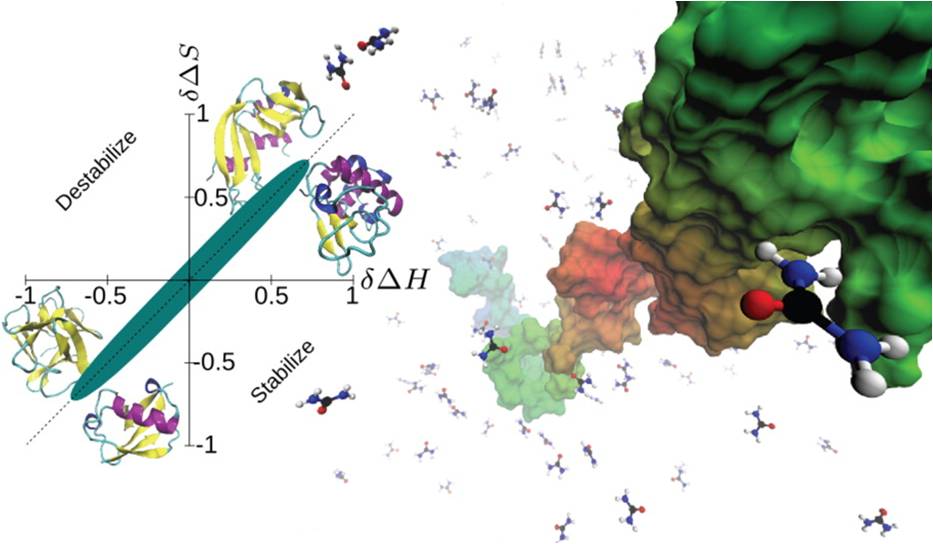
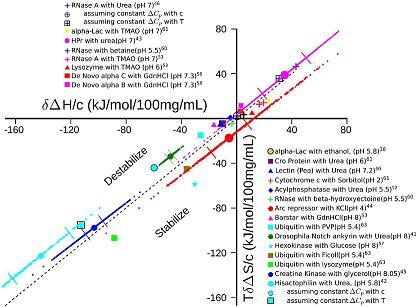
By analyzing a large set of experimental data, we have found significant entropy-enthalpy (SH) compensation for the transfer of a diverse set of two-state folding proteins from water into water containing a diverse set of cosolutes.
These cosolutes include osmolytes, denaturants, ions, crowding polypeptides... basically just about anything you can throw in solution with the protein.
 |
 |
This effect occurs at the melting temperatures of the various proteins, an
|
|
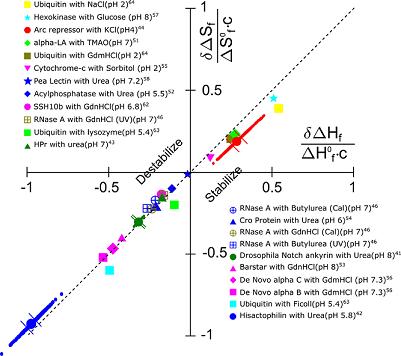
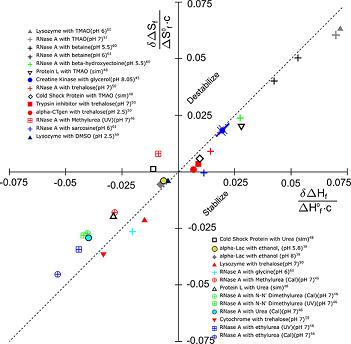
Another confounding problem is that the statistical errors in the entropy and enthalpy almost conspire to destroy the significance of the effect of entropy-enthalpy compensation. See the large scatter in the image below: To account for this, we introduced a new Monte Carlo method to estimate the experimental uncertainty in the thermodynamic data. We then used this method along with bootstrapping to show that SH compensation is statistically significant, in spite of the large, correlated scatter in the data. |
|
An Effective Solvent Theory
We've simulated an all-atom Go model of Trp-cage protein using discontinuous molecular dynamics in an explicit minimal solvent, with a single, contact-based interaction energy between protein and solvent particles. An effective denaturant or osmolyte solution was constructed by making the interaction energy attractive or repulsive. A statistical mechanical equivalence can be demonstrated between this effective solvent model and models in which proteins are immersed in solutions consisting of water and osmolytes or denaturants.
Analysis of these studies yielded the following conclusions: 1), Osmolytes impart extra stability to the protein by reducing the entropy of the unfolded state. 2), Unfolded states in the presence of osmolyte are more collapsed than in water. 3), The folding transition in osmolyte solutions tends to be less cooperative than in water, as determined by the ratio of van ’t Hoff to calorimetric enthalpy changes. The decrease in cooperativity arises from an increase in native structure in the unfolded state, and thus a lower thermodynamic barrier at the transition midpoint. 4), Weak denaturants were observed to destabilize small proteins not by lowering the unfolded enthalpy, but primarily by swelling the unfolded state and raising its entropy. However, adding a strong denaturant destabilizes proteins enthalpically. 5), The folding transition in denaturant-containing solutions is more cooperative than in water. 6), Transfer to a concentrated osmolyte solution with purely hard-sphere steric repulsion significantly stabilizes the protein, due to excluded volume interactions not present in the canonical Tanford transfer model. 7), Although a solution with hard-sphere interactions adds a solvation barrier to native contacts, the folding is nevertheless less cooperative for reasons 1–3 above, because a hard-sphere solvent acts as a protecting osmolyte.
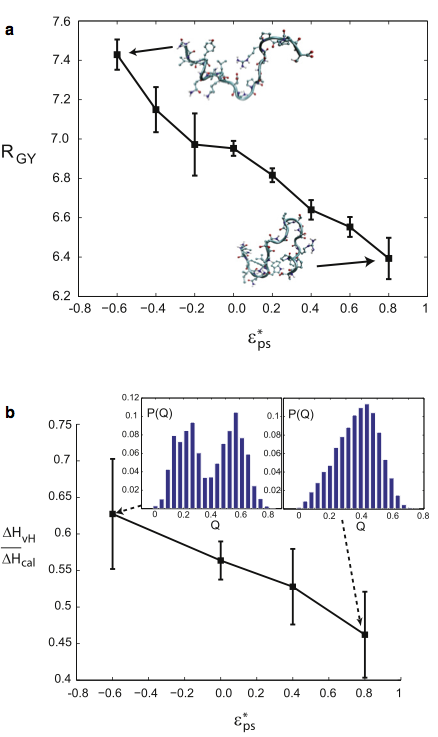 |
The radius of gyration of the unfolded ensemble decreases as the solution around a protein becomes more osmolytic (less denaturing). Also, the cooperativity of the folding transition decreases as the solution around a protein becomes more osmolytic.
|
See Also:
Linhananta A, Hadizadeh S, Plotkin SS, "An effective solvent theory connecting the underlying mechanisms of osmolytes and denaturants for protein stability" Biophys J.100, 459–468 (2011)
Mills EA, Plotkin SS "Protein Transfer Free Energy Obeys Entropy-Enthalpy Compensation" J. Phys. Chem. B. (2015) http://pubs.acs.org/doi/abs/10.1021/acs.jpcb.5b09219
Home | Research | Publications | Prospective Students | Teaching | Contact | Physics | Resources || Fun |
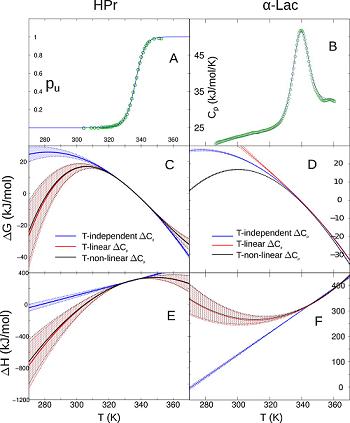 d as well, at lab temperature. To show this, we had to be careful in how we extracted thermodynamic parameters from experimental data.
d as well, at lab temperature. To show this, we had to be careful in how we extracted thermodynamic parameters from experimental data.