


Next: Partition function and the
Up: Boltzmann statistics
Previous: The zeroth law of
Consider now a system in contact with a heat bath, or reservoir.
System 1 is the one we are interested in, and we want to find the
probability P(E1) that it has energy E1. We assume that system
2 is much larger than 1, so that
E1<< E=E1+E2.
Another way
of putting this is to say that the heat capacity C2 of system
2 is very large. We assume that all compatible
microstates are equally likely. We have
From the definition of entropy
We expand in a Taylor series
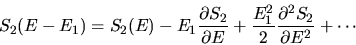 |
(13) |
With T the temperature of the heat bath and C its heat capacity,
the partial derivatives are given by
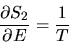 |
(14) |
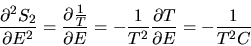 |
(15) |
Since E1<<TC we neglect the last term in
(![[*]](cross_ref_motif.gif) ) giving
) giving
where we define
 . We conclude
. We conclude
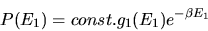 |
(16) |
The factor
 is the Boltzmann factor.
When a system is in contact with a heat bath at a certain temperature,
all possible microstates of the system are no longer equally likely.
Instead, the Boltzmann factor acts as a weight factor biasing the
distribution towards states with lower energy.
is the Boltzmann factor.
When a system is in contact with a heat bath at a certain temperature,
all possible microstates of the system are no longer equally likely.
Instead, the Boltzmann factor acts as a weight factor biasing the
distribution towards states with lower energy.



Next: Partition function and the
Up: Boltzmann statistics
Previous: The zeroth law of
Birger Bergersen
1998-09-14
![[*]](cross_ref_motif.gif) ) giving
) giving